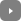An Oak Ridge National Laboratory as part of a team searching for an inexpensive alternative to platinum catalysts, turned to carbon, developing multi-walled carbon nanotube complex that consists of cylindrical sheets of carbon.
Led by Stanford University’s Hongjie Dai, the team’s newly developed carbon nanotube material could help lower the cost of fuel cells, catalytic converters and similar energy-related technologies by delivering a substitute for expensive platinum catalysts.
Platinum has long been prized for its ability to spur key chemical reactions in a process called catalysis, but at more than $1,400 ± an ounce at this writing, its high price is a limiting factor for applications like fuel cells, which rely on the metal.
The cylindrical sheets of carbon are built up to multi-walled carbon nanotube complex. Next the outer wall of the complex is partially “unzipped” with the addition of ammonia. That’s when the new material was found to exhibit catalytic properties comparable to platinum. That was too easy and good to be true.
The researchers suspected that the complex’s properties were due to added nitrogen and iron impurities. However, they couldn’t verify the material’s chemical behaviour until ORNL ‘microscopists’ made images of the unzipped tubes on an atomic level.

Iron and Nitrogen Atoms in the Carbon Nanotube Complex.
Team member Juan-Carlos Idrobo of ORNL offers a brief overview of the procedure, “With conventional transmission electron microscopy, it is hard to identify elements. Using a combination of imaging and spectroscopy in our scanning transmission electron microscope, the identification of the elements is straightforward because the intensity of the nanoscale images tells you which element it is. The brighter the intensity, the heavier the element. Spectroscopy can then identify the specific element. ”

Carbon Nanotube Complex with the Iron Atoms Circled in Red.
The ORNL microscopic analysis confirmed that the nitrogen and iron elements were indeed incorporated into the carbon structure, causing the observed catalytic properties similar to those of platinum. The next step for the team is to understand the relationship between the nitrogen and iron to determine whether the elements work together or independently.
The team’s findings are published in Nature Nanotechnology as “An Oxygen Reduction Electrocatalyst Based on Carbon Nanotube-Graphene Complexes.”
Now the new catalyst isn’t as broadly applicable as platinum, it can act as an oxygen reduction reaction electrocatalyst in both acidic and alkaline solutions. The team by design or happenstance, which isn’t made clear, learned a unique oxidation condition partially unzipped the outer walls of the few-walled carbon nanotubes creating nanoscale sheets of graphene attached to the inner tubes.
So far as is known, the graphene sheets contain extremely small amounts of iron that originated from nanotube growth seeds, and nitrogen impurities, which facilitate the formation of catalytic sites and boost the activity of the catalyst.
Of considerable importance is while the graphene sheets formed from the unzipped part of the outer wall of the nanotubes are responsible for the catalytic activity, the inner walls remain intact and retain their electrical conductivity, which facilitates charge transport during electrocatalysis.
It’s all rather neat. The outstanding question may be what other ‘impurities’ might be applied to arrive at other goals. Those inner walls retaining electrical conductivity are sure incentives for much more research.
By. Brian Westenhaus
Source: Carbon May Substitute for Platinum as a Catalyst