The greenhouse gas carbon dioxide can be converted into useful hydrocarbons by electrolysis. The design of the electrolysis cell is crucial in this process. The so-called zero-gap cell is particularly suitable for industrial processes. But there are still problems: The cathodes clog up quickly.
The team’s research paper has been published in Nature Communications.
The combustion of oil, coal or natural gas produces carbon dioxide, or CO2. This accused greenhouse gas is said to be a major driver of global warming, but it is also a raw material. It is technically possible to convert CO2 into useful carbon compounds, a process which requires energy, water, suitable electrodes and special catalysts.
CO2 can be electrochemically converted to carbon monoxide, formate or methane, but also to ethylene, propanol, acetate and ethanol. However, industrial processes must be designed to be highly selective and extremely efficient to produce only the desired products and not a mixture of products.
Converting CO2 back into fuel
Dr Matthew Mayer, leader of the Helmholtz Young Investigator Group “Electrochemical Conversion” at HZB explained, “By electrolytically reducing CO2 to useful hydrocarbons, we can produce new fuels without using fossil resources. We thus are putting the CO2 back into the cycle, just like recycling.” The electrical energy for the electrolysis can be provided by renewable energy from wind or solar, making the process sustainable.
The zero-gap cell: a sandwich of many layers
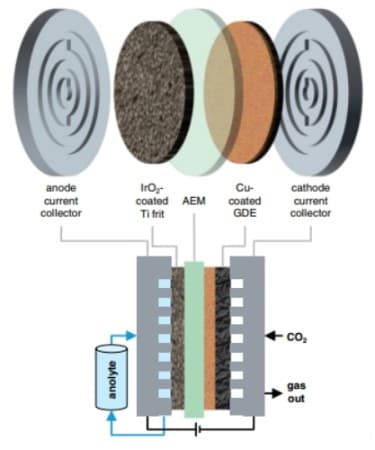
The architecture of the “zero-gap” electrolysis cell. Image Credit: Helmholtz-Zentrum Berlin © HZB. Click the press release link for more info and images.
From school, we know electrolysis can be done in a simple beaker of water; a further development of this is the H-cell, which is shaped like the letter H. However, such cells are not suitable for industrial use. Instead, industrial electrolysers are designed with a sandwich architecture consisting of several layers: On the right and left are the electrodes that conduct the current and are coated with catalysts, a copper-based gas diffusion layer that lets in the CO2 gas, and a separation membrane. The electrolyte (here supplied at the anode and called anolyte) consists of dissolved potassium compounds and allows ions to move between the electrodes. The membrane is designed to allow negatively charged ions to pass through and to block positively charged potassium ions.
The problem: potassium crystals
Nevertheless, potassium ions from the electrolyte pass through the membrane and form tiny crystals at the cathode clogging the pores. “This shouldn’t happen,” says Flora Haun, a PhD student in Matthew Mayer’s team. Using scanning electron microscopy and other imaging techniques, the scientists were able to study the process of crystal formation at the cathode in detail. “With energy-dispersive X-ray analysis, we were able to locate the individual elements and show exactly where potassium crystals were forming,” Flora Haun explained.
The more potassium the electrolyte contains, the more the cathode becomes clogged, the investigations showed. But there is no simple way to solve the problem: reducing the potassium concentration is good on the one hand, but bad on the other, since the reaction equilibrium also shifts: instead of the desired ethylene, carbon monoxide is produced.
The electrolyte is the key
Dr. Gumaa El Nagar, a postdoctoral researcher in the team said, “The most important observation is that cations can still penetrate the anion exchange membrane, but to an extent that depends on the concentration of the electrolyte. And that with the concentration of the electrolyte we simultaneously regulate which products are formed from the CO2.”
Dr Mayer noted, “In the next step, we want to use operando and in situ measurements using X-rays to find out in detail how ion migration in the cell affects the chemical reaction processes.”
***
While we’re still not half way to optimal atmospheric CO2 content there is room for some recycling. This would be particularly true where dense emissions are already getting cleaned from other effluents.
This upside is that soon we could be current in the planet’s carbon cycle. As a long term goal that should be the primary. For now though, climate change hysteria is the politically dominant topic. Fear gathers eyes for media and advertisers much more effectively than making sound forecasts and well thought out plans.
This incredibly useful work. Say coal generating plants had the entirety of the CO2 output recycled again into fuels. That would double the carbon’s use before its returned to the plant kingdom. In fact tripling and more could be possible. A very good thing, indeed.
By Brian Westenhaus via Newenergyandfuel.com
More Top Reads From Oilprice.com:
- Chevron To Buy Shale Firm PDC Energy In $6.3-Billion Deal
- Alberta Wildfires Still Sapping Crude Oil Production
- China Is Breaking Records As Its Solar Industry Booms



















At that point, we need the other 1/2 of the finished product -- hydrogen. That is ALSO energetically bereft of value, so we need to pump MORE renewable energy into it, with substantial losses along the way. In the end, we will have used AT LEAST 5 units of renewable electricity to make 1 unit of chemical energy in the form of some organic molecule. And when we burn that molecule, we will lose another 50%-plus of the energy in converting it to motive power (as vehicle fuels) or converting it back to electricity (as power plant fuels).
Meanwhile the renewable energy that was used for this could have been used directly, transported, and even stored FAR more efficiently. I am a "new technology" guy, I believe we need to convert our economy to renewable energy, and I don't have a personal financial or professional stake in any particular alternative. But I believe that CO2 capture, especially from the air, and its conversion to fuels is the WORST kind of wasteful approach.